 PDF(1890 KB)
PDF(1890 KB)


球状纳米二氧化钛/石墨烯复合材料的合成及导电性能
刘琳, 李莹, 鄂涛, 杨姝宜, 姜志刚, 许丽岩, 张天琪
材料工程 ›› 2019, Vol. 47 ›› Issue (8) : 97-102.
 PDF(1890 KB)
PDF(1890 KB)
 PDF(1890 KB)
PDF(1890 KB)
球状纳米二氧化钛/石墨烯复合材料的合成及导电性能
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Synthesis and electrical conductivity of spherical nano-TiO2/graphene composites
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}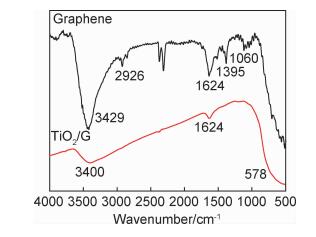
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |