 PDF(1042 KB)
PDF(1042 KB)


 PDF(1042 KB)
PDF(1042 KB)
 PDF(1042 KB)
PDF(1042 KB)
质子交换膜燃料电池关键材料的研究进展
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Research progress of key materials in proton exchange membrane fuel cell
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}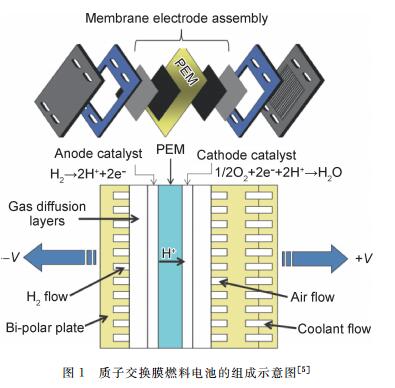
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |