 PDF(2198 KB)
PDF(2198 KB)


 PDF(2198 KB)
PDF(2198 KB)
 PDF(2198 KB)
PDF(2198 KB)
SO42-浓度对304不锈钢在NaCl 溶液中点蚀行为影响的研究
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Effect of SO42- Concentration on 304 Stainless Steel Pitting Corrosion in NaCl Solution
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}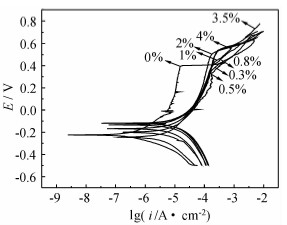
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |